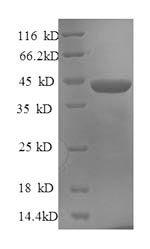
Purity
Greater than 90% as determined by SDS-PAGE.
Research Area
Transcription
Alternative Names
Alternative splicing factor 1; Alternative-splicing factor 1; arginine/serine-rich 1; ASF 1; ASF; ASF-1; ASF1; FLJ53078; MGC5228; P33 subunit; Pre mRNA splicing factor SF2 P33 subunit; pre-mRNA-splicing factor SF2; Serine/arginine-rich splicing factor 1; SF2; SF2P33; SFRS1; Splicing factor 2 alternate splicing factor; Splicing factor 2; Splicing factor; Splicing factor arginine/serine rich 1; SR Splicing factor 1; SRp30a; srsf1; SRSF1_HUMAN
Species
Homo sapiens (Human)
Expression Region
2-248aa
Target Protein Sequence
SGGGVIRGPAGNNDCRIYVGNLPPDIRTKDIEDVFYKYGAIRDIDLKNRRGGPPFAFVEFEDPRDAEDAVYGRDGYDYDGYRLRVEFPRSGRGTGRGGGGGGGGGAPRGRYGPPSRRSENRVVVSGLPPSGSWQDLKDHMREAGDVCYADVYRDGTGVVEFVRKEDMTYAVRKLDNTKFRSHEGETAYIRVKVDGPRSPSYGRSRSRSRSRSRSRSRSNSRSRSYSPRRSRGSPRYSPRHSRSRSRT
Note: The complete sequence including tag
sequence, target protein sequence and linker sequence could be provided upon request.
Protein Length
Full Length of Mature Protein
Tag Info
N-terminal 6xHis-SUMO-tagged
Form
Liquid or Lyophilized powder
Note: We will preferentially ship the format that
we have in stock, however, if you have any special requirement for the format, please remark your
requirement when placing the order, we will prepare according to your demand.
Buffer
If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol.
Note: If you have any special requirement for the
glycerol content, please remark when you place the order.
If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer,
6% Trehalose, pH 8.0.
Reconstitution
We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20°C/-80°C. Our default final concentration of glycerol is 50%. Customers could use it as reference.
Storage Condition
Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw
cycles.
Shelf Life
The shelf life is related to many factors, storage state, buffer ingredients, storage temperature
and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized
form is 12 months at -20°C/-80°C.
Lead Time
3-7 business days
Notes
Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
Datasheet & COA
Please contact us to get it.
Description
The region for expressing recombinant Human SRSF1 contains amino acids 2-248. This SRSF1 protein is theoretically predicted to have a molecular weight of 43.6 kDa. Expression of this SRSF1 protein is conducted in e.coli. The SRSF1 coding gene included the N-terminal 6xHis-SUMO tag, which simplifies the detection and purification processes of the recombinant SRSF1 protein in following stages of expression and purification.
Serine/arginine-rich splicing factor 1 (SRSF1), also known as SF2/ASF, is a multifunctional RNA-binding protein involved in pre-mRNA splicing regulation. SRSF1 plays a crucial role in constitutive and alternative splicing by binding to exonic splicing enhancer elements. Its main function is to facilitate the recognition of splice sites during spliceosome assembly, influencing the inclusion or exclusion of exons in mature mRNA transcripts. Beyond splicing, SRSF1 has been implicated in various cellular processes, including mRNA export, translation, and mRNA stability. Aberrant expression or post-translational modifications of SRSF1 are associated with several diseases, including cancer, where it can contribute to oncogenesis by altering the splicing patterns of key genes. Research on SRSF1 continues to uncover its intricate roles in gene expression and its potential as a therapeutic target in various pathologies.